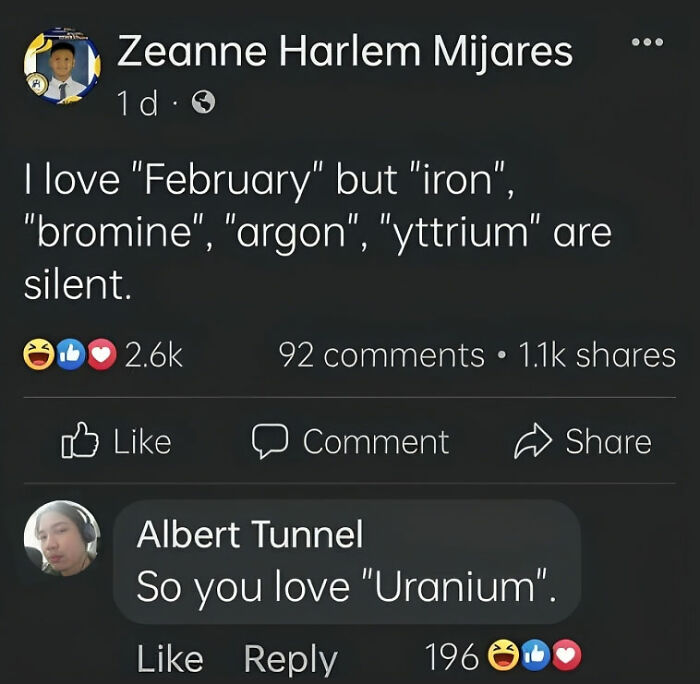
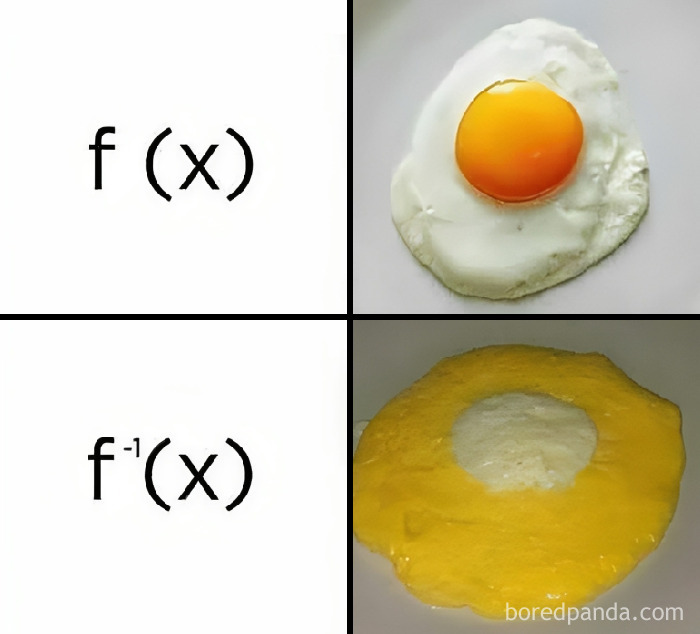
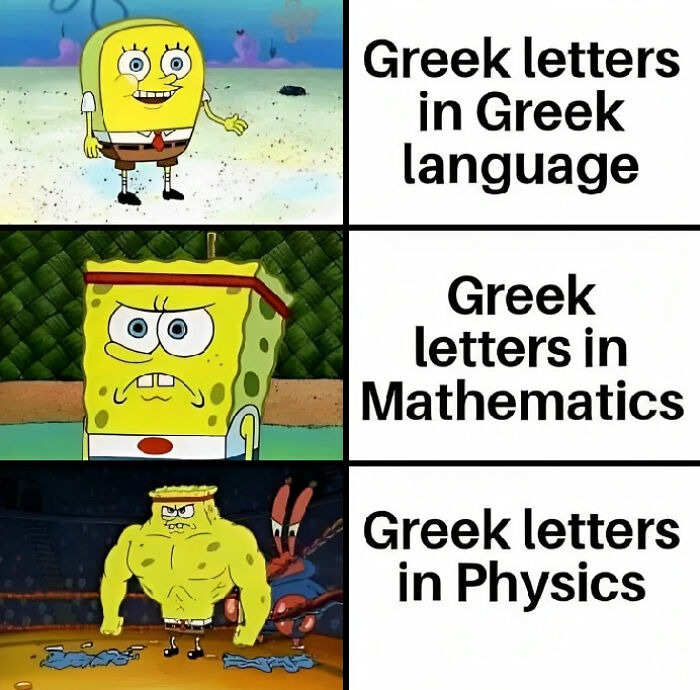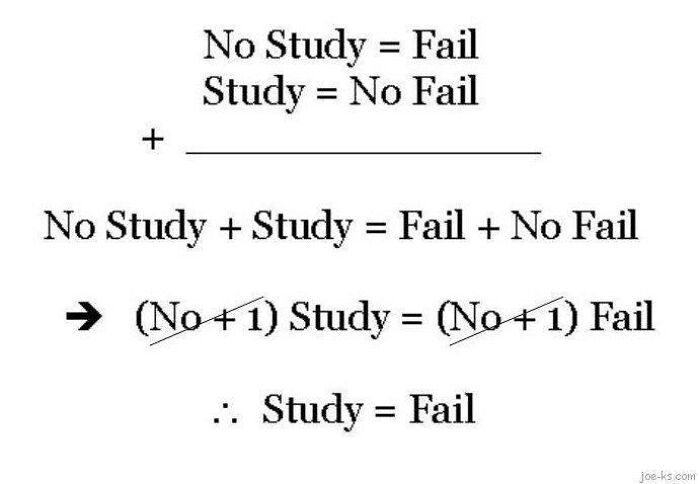Humor helps you remember information far more easily. So when you combine education with entertainment, you create something truly stunning. The ‘Basic Physics’ Facebook group is an extremely popular community that shares witty and relatable science memes.
We’ve compiled a list of the funniest ones to share with you, Pandas, to prove that science doesn’t have to be boring at all. Scroll down for the best memes, as featured on the group, and remember to upvote the ones you liked the most. Bored Panda has reached out to the team running ‘Basic Physics’ on Facebook, and we’ll update the article as soon as we hear back from them.
This post may include affiliate links.
At the time of writing, the ‘Basic Physics’ group has a jaw-dropping 2.1 million members. The community has grown by leaps and bounds since it was founded in early October of 2018. The members are very active and in the last month alone, they’ve made 286 new posts.
Meanwhile, the team running ‘Basic Physics’ is fairly large. It’s made up of 7 administrators, as well as 9 moderators. It makes sense to have so many people from all over the world on the team, considering the massive size of the group itself.
Not exactly correct: As various mutations spread, the one that spreads the best is the one that limits the host's socialization the least. Making you die is the least successful. Immediately laying you up is still pretty bad. Letting you spread the microbe for a few days and then making you stay home sick works pretty well, but is problematic because it has to avoid triggering the immune system. The most successful virus spreads without ever making people sick.
The team managing the community calls it a ‘scientific group’ and claims that its mission is to present the public with highly educational posts about physics and its various branches.
According to the group founders, these include classical mechanics, thermodynamics and statistical mechanics, electromagnetism and electronics, relativity, quantum mechanics, string theory, quantum gravity, loop quantum gravity, optics, atomic, molecular, and optical physics, condensed matter physics, high energy/particle physics and nuclear physics.
Then I’m irrational (yay 2 of my favorite numbers are pi and phi)
However, not all posts have to be super serious and focused just on physics. General memes about science and math are welcome, too.
According to the administrators and moderators, as the community continues to expand, it’s important, more now than ever before, to create a welcoming environment while also staying true to the group’s original vision.
The team running the entire project stresses that it’s vital that members are respectful of one another. They urge Facebook users to treat each other like they themselves would want to be treated. Especially during online arguments. “Healthy debate is natural, but humility is also necessary,” the team writes.
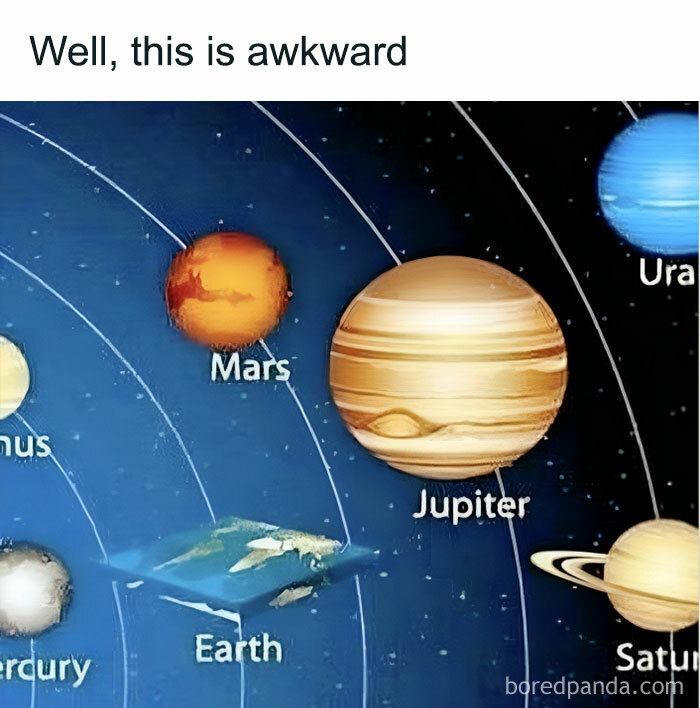
It's true. We have no reason to suppose a dilophosaurus would spit poison at Newman, or have a colorful neck frill. But that was put into Jurassic Park because we have little reason to suppose that they did NOT.
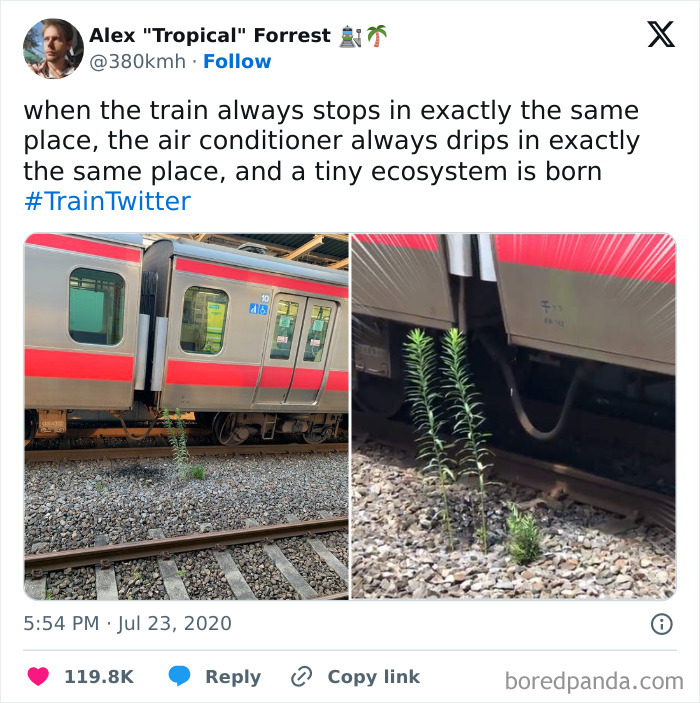
And then there are my house plants. 'oh you are going out for a day, I guess I will die.'
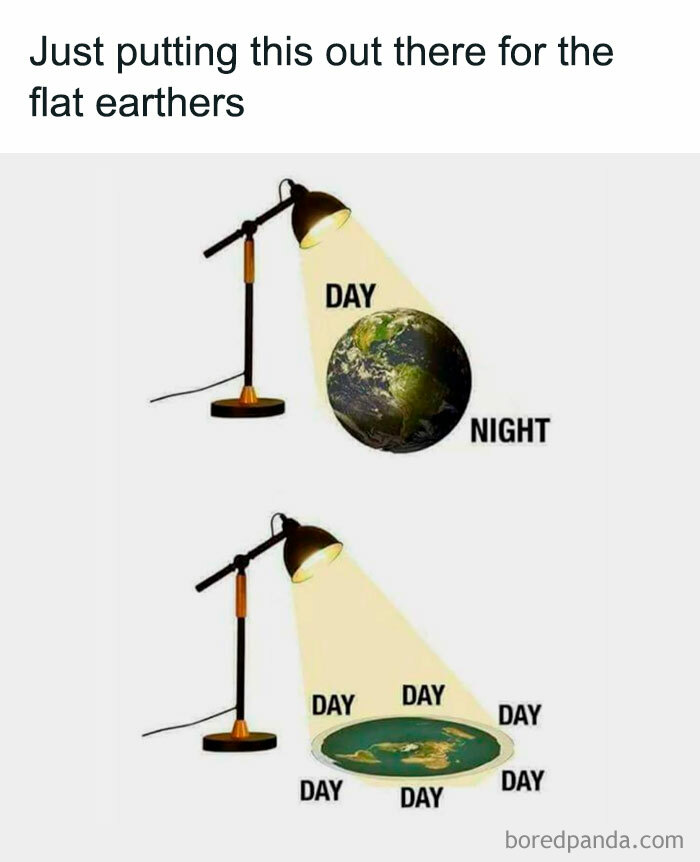
I really like flat earthers because they are a group that is completely socially acceptable to make fun of. (In a kind, big-hearted way, of course…)
The ‘Basic Physics’ admins and mods also want every member to feel safe. “Bullying of any kind is not allowed and any derogatory comments related to things like race, religion, culture, sexual orientation, gender, or identity will not be tolerated,” they explain that there’s no tolerance for intolerance.
On top of that, there’s a certain expectation that all members give back more to the community than they get from it. That means that everyone should avoid spamming the group, posting irrelevant links, or promoting themselves. “To be a part of this group, there will be a need to establish mutual trust. Authentic, passionate discussions improve the group, but these discussions can also be sensitive and personal. Content shared in a group must remain in the group.”
I’ve always wanted one of these things, they’re entertaining to me
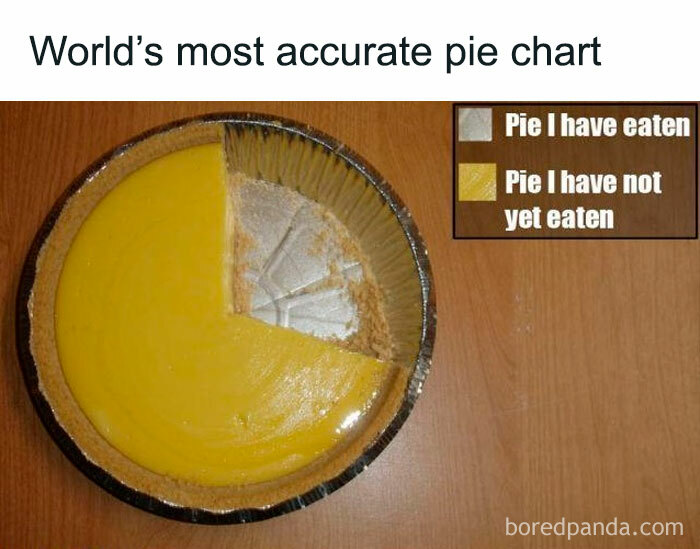
What about the third segment representing the crumbs? Neither eaten, nor to be eaten.
Though it’s impossible to predict what memes will go viral, some posts tend to do better than others on social media. One key aspect of a quality meme will be its relatability.
The more your chosen audience can resonate with your posts, the more likely people are to engage with them and then share them with their own friends and followers. Throw in a bunch of humor and you create an even more powerful concoction.
On the flip side, if your memes are very niche or overly complicated, they might not get a lot of attention. Of course, you should definitely keep in mind that there’s definitely nothing wrong with making and sharing niche memes. If you enjoy doing that as a content creator, then more power to you!
But you shouldn’t expect overnight internet fame if you focus on jokes that are about a small subsection of a more general branch of physics. Then again, internet popularity sometimes comes when someone least expects it…
On top of that, making your memes ‘evergreen’ can give them a longer shelf life than if they were entirely based on recent news. General memes about science or what it’s like to be a researcher can remain relevant for years and potentially even decades to come than posts that require a lot of additional context to get.
To neutralize 0.8 mL of sulfuric acid (H2SO4) at STP, you'd need about 0.8 moles of sodium bicarbonate (NaHCO3) based on the balanced chemical equation: H2SO4 + 2NaHCO3 → Na2SO4 + 2H2O + 2CO2 Each mole of H2SO4 requires 2 moles of NaHCO3. So, 0.8 mL of H2SO4 is approximately equivalent to 0.8 moles when considering the negligible volume at STP.
Load More Replies...That question can’t be answered because there’s not enough info
Just ask AI lol: To neutralize 0.8 mL of sulfuric acid (H2SO4) at STP, you'd need about 0.8 moles of sodium bicarbonate (NaHCO3) based on the balanced chemical equation: H2SO4 + 2NaHCO3 → Na2SO4 + 2H2O + 2CO2 Each mole of H2SO4 requires 2 moles of NaHCO3. So, 0.8 mL of H2SO4 is approximately equivalent to 0.8 moles when considering the negligible volume at STP.
Load More Replies...I dont understand this, so based on my understandig of sosiaty, im guessing he is asking how to dissolve a body Please correct me if im wrong
the fact that i can answer this question but refuse to to even bother to try🤦♀️😬
Do you know the way back when ‘ pink’ was actually green? But eventually became the color pink as we know it today. Go figure.
Well... huh. I'm going to need a chemistry lesson. What is a mole and how does it differ from a molecule? Does this need to be exact? Are we measuring using pH scale? What is STP? Please define. If we're using pH that would imply the sodium bicarbonate is alkaline, yes? I never took chemistry so I would probably need instructions and/or assistance to do the experiment/ math required. If course now in curious. How many moles?
I would like to see the message above the top right and the message below the bottom left. Cropping tells a very specific story...
0.03 moles to be exact Volume of sulphuric acid: 0.8ml Density of sulphuric acid: 1.8g/ml Molar mass of sulphuric acid: 98.079g/mol Reaction formula: 2NaHCO3(aq)+H2SO4(aq) → Na2SO4(aq)+2CO2(g)+2H2O(l) Note the 2 to 1 ratio. Moles needed: 0.8×1.8/98.079×2 = 0.029 mole A mole is (or at least used to be) the number of carbon 12 atoms in 12 grams of carbon 12, about 6.02214076×10^23 .
Sulfuric acid is a solution of hydrogen sulfate dissolved in water. You can't solve the mentioned equation without knowing either the mass or concentration of the solution.
Load More Replies...I got this! To neutralize 0.8 mL of sulfuric acid (H2SO4) at STP, you'd need about 0.8 moles of sodium bicarbonate (NaHCO3) based on the balanced chemical equation: H2SO4 + 2NaHCO3 → Na2SO4 + 2H2O + 2CO2 Each mole of H2SO4 requires 2 moles of NaHCO3. So, 0.8 mL of H2SO4 is approximately equivalent to 0.8 moles when considering the negligible volume at STP.
The maximum concentration of sulfuric acid is 18 mol/l. Unless the solution is superconcentrated, this means it has a maximum of .0144 Mol of molecules to neutralize. Since the neutralization reaction requires two sodium bicarbonate, the maximum number of moles of bicarbonate needed is .0288. However, since the concentration of the acid or the mass of the solution isn't provided, this problem has no single answer. The actual number required could be as few as two molecules
Load More Replies...If you came up with an answer, you assumed information that wasn't provided.
Load More Replies...So, dear Pandas, which of these science memes did you like the most? Were there any that made you chuckle harder than you’d care to admit? Were there any posts that you didn’t quite get because they needed more knowledge in physics? We’d love to hear your thoughts, so when you’re done enjoying our post, be sure to drop by the comment section to share your opinions!
Some days I feel like I could be a meme for Schrödinger’s Sanity… Broken? Unbroken?
Yet it feels great when it turns out you had the right answer all along and everyone else was just fighting over wrong answers
Ha! I think this one’s a typo. It was Schrödinger who had the cat. 😎😎😎 ETA: You guys are great! Thanks for not down voting me!
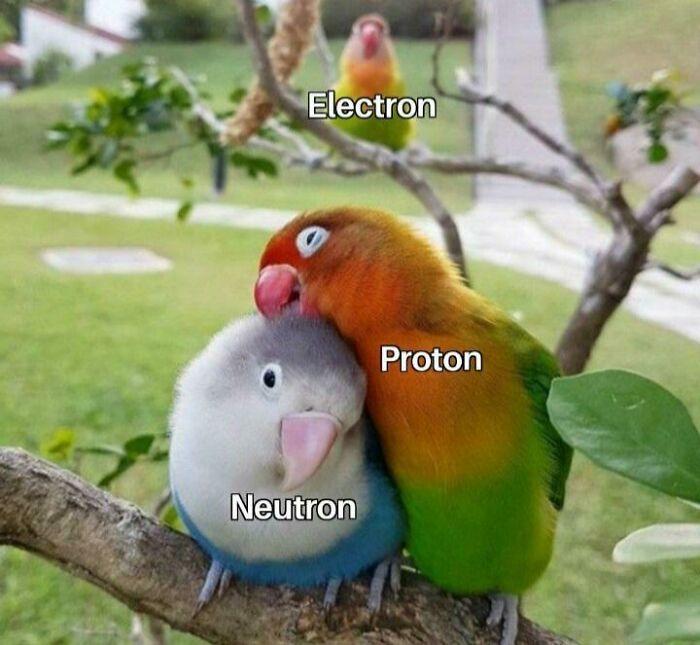
Molecules share them. Quite friendly. It's the ions that are so mean.
*mixes water-explosive with poison gas… gets high blood pressure*
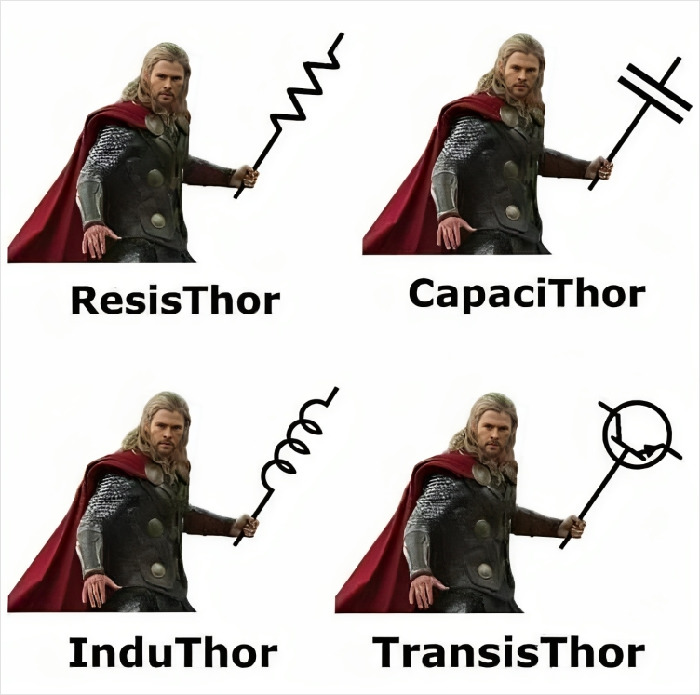
Wouldn't it be awesome if the electricity was created by a Thorium reactor?
I laughed and laughed :) . Best topic I've seen in a long time on BP. More of these, please!
I guess the headline for this meme article is "80 memes" but only 50 real ones is the final joke.
At the end of the list there's a link to the full 80. It's itty bitty so you kinda have to look for it.
Load More Replies...Then ther were those commenters that had absolutely no sense of humor and didn't get it was a joke.
I LOVED EVERY ONE!!!! and did not even resent being unable to stop . . .
I LOVED ALMOST EVERY ONE!!!!!!!!! and did not resent starting and being unable to stop . . .
I laughed and laughed :) . Best topic I've seen in a long time on BP. More of these, please!
I guess the headline for this meme article is "80 memes" but only 50 real ones is the final joke.
At the end of the list there's a link to the full 80. It's itty bitty so you kinda have to look for it.
Load More Replies...Then ther were those commenters that had absolutely no sense of humor and didn't get it was a joke.
I LOVED EVERY ONE!!!! and did not even resent being unable to stop . . .
I LOVED ALMOST EVERY ONE!!!!!!!!! and did not resent starting and being unable to stop . . .

 Dark Mode
Dark Mode  No fees, cancel anytime
No fees, cancel anytime